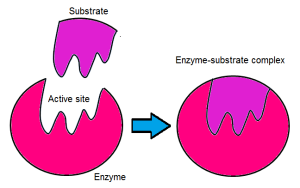
Enzymes are biological catalysts which speed up all processes via a chemical reaction by providing an alternative pathway with lower activation energy. Most enzymes are proteins (but not all proteins are enzymes) and some are RNA molecules. They are large molecules which consist of amino acids however, only a small portion of the enzymes play a role in the catalysis of the biochemical reactions. This portion is called the active site. The enzymes act upon molecules called substrates and this reaction occurs in the active site. Hence, an enzyme-substrate complex is formed. This bonding is due to hydrogen and hydrophobic bonds.
E + S ⇌ ES → E + P
E= Enzyme
S= Substrate
P= Product
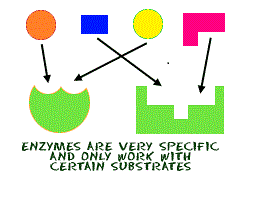
Enzymes work to lower the activation energy. They are specific and can only work with certain substrates. Denaturation of an enzyme can occur due to high temperatures and pH values, however when an enzyme becomes denatured the shape of the active site is altered and the substrate molecule is no longer able to fit. The activation energy is the minimum amount of energy required for the reaction to occur. When the activation energy is lowered, the more substrate molecules will be converted to product per unit time, hence there is more energy and the reaction occurs at a faster rate.
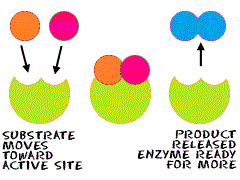
When an enzyme and substrate bind at the active site, a process called catalysis occurs. Catalysis occurs when the substrate is altered and can be broken down or bound to another molecule. This allows for the creation of a new molecule. The enzyme then releases the substrate and returns to its normal state however, the initial molecule has been modified. It is now called the product.
Enzymes exhibit several degrees of specifity. These include:
- Relative Specificity– where there is an interaction of a molecule with several substrates but with different affinities;
- Absolute Specificity– refers to the situation whereby the enzyme catalyzes one reaction;
- Group Specificity– occurs when the enzyme acts only on molecules with specific functional groups, for example, the amino and phophate groups;
- Linkage Specificity– occurs when the enzyme acts on a specific type of chemical bond despite the molecular structure;
- Stereochemical Specificity– occurs when the enzyme is acting on a steric or optical isomer.
Enzyme regulation occurs due to inhibitors. These inhibitors are molecules which are used to alter the catalytic reactions occurring, thus slowing down the reaction. Inhibitors bind reversibly or irreversibly. Reversible inhibitors are bound non-covalently and can be detached by the enzyme easily by dilution or dialysis. These reversible inhibitors include:
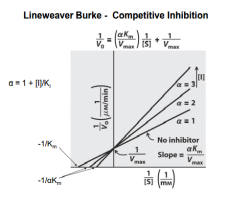
- Competitive inhibitors- the inhibitor and the substrate resemble each other; the inhibitor binds to the active site of the enzyme; Km will increase whereas the Vmax will not change.
- Uncompetitive inhibitors- the inhibitor does not resemble the substrate, the inhibitor binds only to the substrate-enzyme complex; Km and Vmax decreases to the same amount.
- Non-competitive inhibitors- is a form of mixed inhibition; the inhibitor does not resemble the substrate. Vmax decrease, Km is unaffected.
- Mixed inhibitors – the inhibitor can bind to the enzyme at similar times as the enzyme’s substrate; Vmax decreases, while Km may either increase OR decrease.